Part II
5 Study Designs Commonly used in Epidemiology
Study Designs Commonly used in Epidemiology
Learning Objectives
By the end of this chapter, the learner will be able to
- Describe the most common research study designs used in epidemiology
- Differentiate between Non-experimental Observational studies, and Experimental/Interventional epidemiological studies
- Differentiate among individual and population based studies, and also between observational, descriptive and analytic studies.
- Understand the use of randomization in experimental studies such as clinical trials, and other types of experimental field trials.
Introduction to the chapter
This chapter will present the most commonly used epidemiological study designs,listing main characteristics and then, focusing on their benefits, strengths, weaknesses, and uses in public health.
Most epidemiologists are trained to do their investigation based on a series of designs called, Study Designs. The study Designs commonly used in epidemiology are based on several premises, but a series of questions can help the investigator to decide what design best fits its needs, some of these questions are, what types of study designs are there? How and when we use specific type of study designs? Which study design is the most appropriate to use in certain investigations? The list of questions could continue but it is important to generate these questions in order to arrive to a decision of what fits better the investigators needs. Also, investigators need to be familiar with these study designs so they can use them when needed.
What are study designs in epidemiology? Study designs refer to the different approaches mainly used to conduct research for investigative purposes. They are called, ‘designs’ because they represent a specific manner of conducting the research process, which is mainly based on the scientific method. Study designs are more of a framework to guide the researcher in the process. [1] And, although basically all research process starts with a research question, there is need to follow a process that will convert this research question into a hypothesis, and then, to a real life situation, or, scenario that need to be framed in order to arrive to valid conclusions. [2] In other words, study designs assists the researcher providing a type of road map that will help to not get lost. It is easy to get lost, especially when complex health phenomena is studied/researched, but the study design is expected to assist in providing direction. [3] In sum, study designs are road maps, or, frameworks that assist in the research/investigative process.
It is also probably useful to mention that some of the study designs mentioned here, are not only used in the sciences of epidemiology, they are used by other areas of study, especially those areas that belong to the social sciences, including public health, but also mathematics, statistics, and of course, medical sciences. So, this study designs are not unique to the field of epidemiology, but they are highly used in public health, and medical research.
Descriptive versus Analytic
In a broadly manner, epidemiologic study designs can be divided into two broad categories: 1) Descriptive and 2) Analytic.
Descriptive studies as the word implies, ‘describe’ situations, problems, and other health phenomena (diseases, disorders, health behavior, healthy lifestyles, etc.). These type of study designs are mainly used to generate hypotheses, especially in those cases in which the health issue in study is unknown, or, there is not much information on it, or, simply, because the topic is a ‘new’ problem found in sciences, in this case, social sciences, including epidemiology. On the other hand, Analytic studies, ‘analyze’ the health phenomena, situation, or, problem.
More elements used to distinguish between descriptive and analytic research studies
Since epidemiology is by nature quantitative, the division between descriptive and analytic studies can be also clearly recognized by the type of quantitative methods, in this case, the methodology, data collection, and statistical analyses that are used in the research process. For example, in descriptive study designs, the most common data analysis is the use of ‘descriptive statistics’ such as numbers, percentages, sums (total number of cases), mean, mode, standard deviation, etc. Since, the descriptive study is looking mainly for a ‘description’ of the problem, the use of descriptive analyses suffice for the type of research that mainly intents to generate hypotheses, or, to add more information for future studies.
Overview of Study Designs – the following is a list of study designs:
Descriptive Studies
In this category, the following are the most commonly used/listed:
a) Case Study or, Reports
b) Case Series
c) Cross-sectional studies
d) Ecologic studies
Analytics Studies, since they are more complex, they are subdivided into two additional categories: Observational and Experimental.
Observational studies are mainly represented by the following:
a) Cross-sectional studies – as you probably had noted, this category is shared also with the ‘descriptive’ category, which means that a cross-sectional study can be descriptive, observational, or, analytic.
b) Case- control studies
c) Cohort studies
Experimental studies
In this category, it is customary to include the following:
a) Clinical Trials
b) Community Trials
c) Other forms of experimental research.
The information listed above can be summarized in the following Figure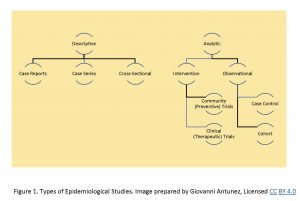 The information has been summarized somehow in publications, such as the ‘Study designs commonly used in epidemiology, from community medicine, study designs, howmed net.[4]
The information has been summarized somehow in publications, such as the ‘Study designs commonly used in epidemiology, from community medicine, study designs, howmed net.[4]
Now, the question is, how do we know what study design to use? As it is seen (listed) above, there is a repertoire of study designs available to the investigator. The use of any of these designs depends on the mainly purpose/reason for the study, and also, the resources, which are mainly financial, and expertise of the researcher team.
How do we choose certain study design from the list of options?
Many times, the answer to this question depends on the purposes/reasons for the research, or, investigation of a certain health phenomena. For example, if we want to conduct a study about a topic/area that is unknown, or, not well-known, and the research question is still a work in process, or, it is clear, but there is need to elaborate a hypothesis/or, hypotheses; then, the most appropriate study design in this case is the descriptive, why? It is because descriptive studies are commonly used for hypotheses generating. Does it mean that there is no other way to conduct the study? Not necessarily, because they may be another way to do the same, but over and over, especially in social sciences, public health, and specifically in epidemiology, descriptive study designs have been used for those purposes, in this case, hypotheses generating.
Another example that refers to the purposes/reasons for the research but that also refers to the financial aspects of medical, and public health research is the cohort study. In this case, if the main reason/purpose of the study is to find a causal relationship, the cohort study is the answer. This type of study design will help overtime to elucidate the associated risk factors, and social health determinants that are related to the health problem that is researched. What is the only reason that stop the use of the cohort study? The main obstacle is financial, cohort studies can be highly costly, since, study subjects are basically follow over a long period of time until the health outcome is developed, as it is the case of for example the cardiovascular disease cohort studies conducted in the United States. More information about cohort studies will be provided/expanded later in the context of this chapter, and the overall book content itself. [5]
Descriptive Study Designs
The Case Studies/Case Series
This study design is commonly used when there is no much information about the case, as it is the example of a recently reported disease, or, a disease that is very rare, so, the investigator wants to share the information with the scientific community, but since there is only one, or, three cases, it is much better to choose this study design, which sometimes become a case series, if a continuation of cases are reported in a limited fashion. The main limitation is that the cases are not necessarily representative of the general population, but the benefits are that the reported case brings an opportunity for future studies on the subject.[6], [7]
In most epidemiological textbooks, the case studies are commonly used by the medical community to report a case, or, a series of cases that usually represent patients suffering from certain diseases. The medical model usually reports cases that are diseased. But the model hardly applied to other health phenomena, and since it is usually oriented to report cases in a limited fashion, there is small use of this design in epidemiology, which focuses on populations at large.
Ecologic Studies
For this study design, the unit of analysis is the group, not the individual. In this case, correlations are obtained between exposures rates and disease rates among different groups, or, populations. Because of the word, ‘ecologic’, ecological studies tend to be confused with ‘environmental’ studies, and this is possible especially if an environmental issue is studied using this design, but in general, ecologic studies refer to the group(s) investigated.
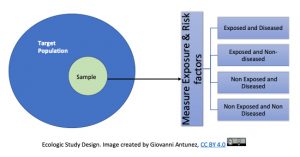
The ecological study provides a setting in which observations made at the group level may not represent the exposure-disease relationship at the individual level, this is called, the ecologic fallacy, which occurs when incorrect inferences about the individual are made from the group level data. [8].
Essentially, this means that inferences from ‘the results of ecological studies can only be applied to the group but not to the individual’. You may said, why? And the answer is because the intention of the ecologic studies is to capture how the health events are affecting the group, the community and not the individual. An example of this, is this study done in alcohol consumption and coronary heart disease (CHD), see the image below that presents the respective information:
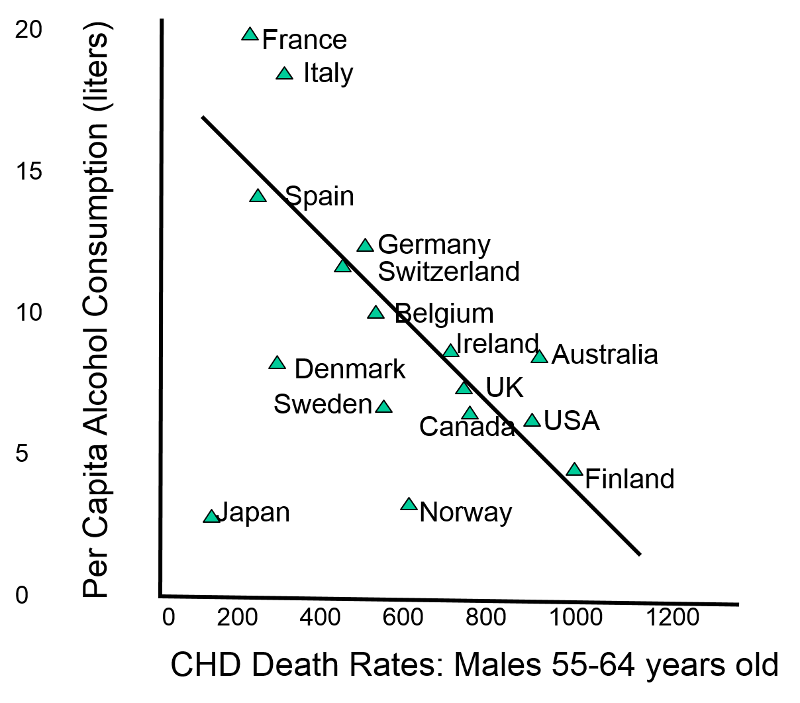 |
| ‘Death rates of coronary heart disease (CHD) in different countries’ , image from PH717 Module 1B |
In the study of the image presented above, the data across countries (the ‘population’ or, ‘group’ mentioned in the definition of ecologic studies) showed that moderate use of alcohol was not beneficial to the heart, on the contrary, it increases the risk for CHD, why? It is the typical case in which what is true at the group or, population level, it is not true to the individual level. Studies done on individuals or, specific groups had shown that moderate alcohol consumption is beneficial to prevent CHD. [9]
As it has been presented in the example above, the ecologic fallacy which is part of the nature of an ecologic study, it is also considered a disadvantage of this type of study design. Another disadvantage is that ecologic studies could make imprecise measurement of exposure and disease. [10].
On the other hand, the following are advantages of ecologic studies, they are quick, simple and less costly than other studies, and their completion is faster compared to other designs used in epidemiology and related sciences. One more advantage is that they can be used (and very useful in this sense) for generating hypotheses, especially when a disease is of unknown etiology.
Common uses of ecologic studies
Specific applications of the ecologic study design has been classified by some authors as the following: geographical comparisons, time trends, migrants, occupation and social class. More details are included below:
Geographical comparisons, which for example can be used to find prevalence of risk factors by comparing incidence or, mortality in two, or, more geographic areas. Time trends, which essentially means to study the fluctuations on the incidence of chronic diseases which tend to change over time. Migrants, the study of migrant groups can be used to identify those factors that are predominantly genetic from those who are environmental, in this case, first or, second generation of an ethnic group maybe affected differently depending on their degree acculturation. Occupation and social class, in this case, it refers on how some morbidity and mortality area associated with certain occupations, and also with the socioeconomic status of the groups working on those type of jobs.[11]
Cross-Sectional Studies
This study is a commonly used design. As a way to understand the most basic principle of a cross sectional study, is to think on the total study population as a pie, in which each percentage represents a section of the pie, then, for study purposes only a piece (or, section) of the pie is investigated. Since in the majority of cases, the characteristics of a population are very similar, choosing to study one portion (section) of the pie (the population) will be representative of the total population. Assuming that we are talking about a study population, not the general population. This analogy takes us to the term, cross (cutting) section (a piece of the pie) study.
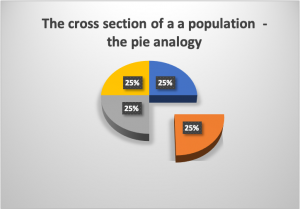 |
Figure 2. The Pie analogy to understand the concept of Cross-Sectional study. Figure prepared by Giovanni Antunez. Licensed CC BY 4.0  |
More about the cross sectional study design
Keeping the above analogy in mind, let’s look at the cross-sectional study in another way, first we draw a single sample from the target population and assess current exposure and disease status on everyone, see image below: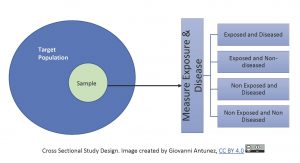
Since cross-sectional studies collect data at a point in time, they are commonly used to calculate prevalence for public health reports, and also for the designing and location of health services in a community. This study design is used frequently, especially when there is not much money to afford another type of study. So, cross sectional studies are very popular not only because they are less costly, but also they are fast to complete. Another great advantage of cross sectional studies is that they are used to generate hypotheses, or, specific research questions about exposure and disease, however, this type of study does not address the issue of temporality, due to being one shot only (one point in time), it does not provide information to know what was first between cause and effect.[12]
Example
An example of this type of study design is, an Australian cross-sectional study on the effects of screen and non-screen sedentary time in adolescents, and how these types of behaviors affect their weight and overall well-being. The study found that although screen sedentary time (SST) is a contributing factor in the amount of fatness observed in school-age children and adolescents, there are two other factors that need to be studied if a significant change is expected to be observed, and these are active lesson breaks in the classroom, or active transport to school. These two last factors are part of what the study called, the NSST or, Non-screen sedentary time. [13] As part of the study design (cross-sectional), some of the study variables are presented in the figure below (taken directly from the published article), see below:
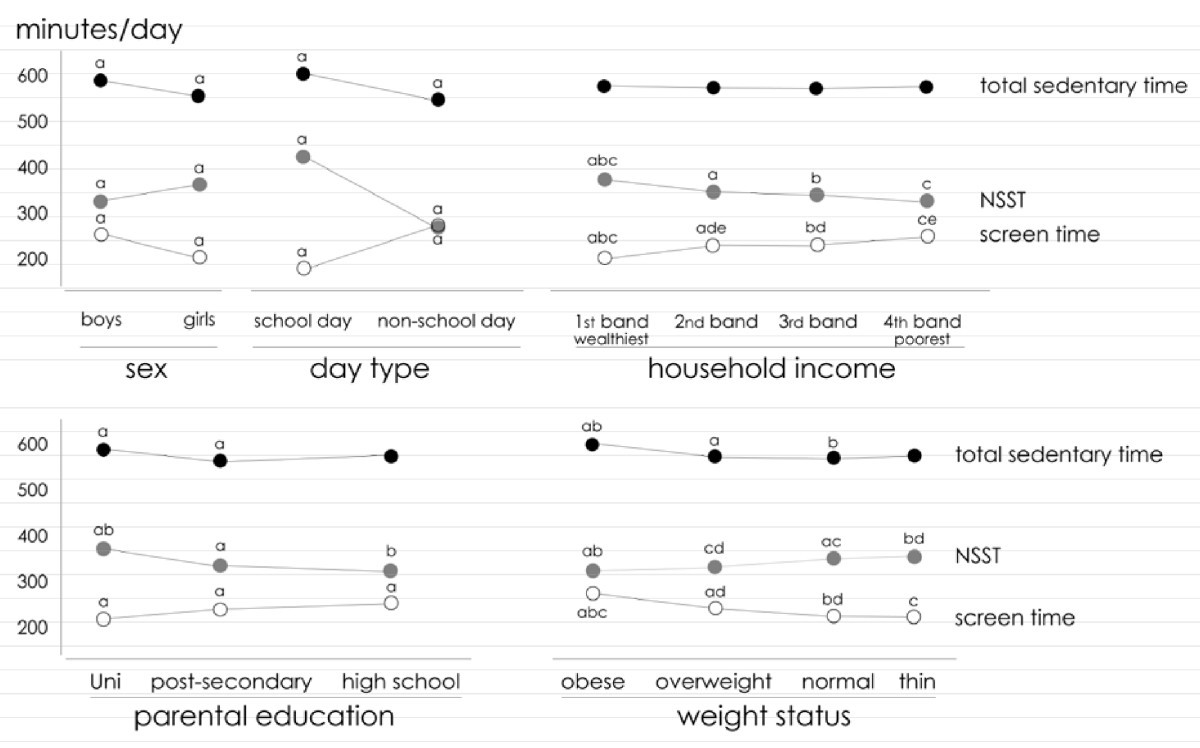 |
| ‘The relationship between non-screen, screen and total sedentary time’ [selected variables] – example of a cross-sectional study, image from the article cited/mentioned above. |
Finally, one of the major benefits of cross sectional studies is they are quick (compared to other type of designs such as the cohort study) to complete, making them very efficient in terms of time and cost.
Case-control studies
This type of study design is a relatively commonly used study, and the main reason is because analysis the exposure and disease event in a retrospective manner (the data has been already collected, and it can be found from medical records, disease registers, and databases of some specific diseases/health problems. Below is a graphical representation on how the cases and control are selected:
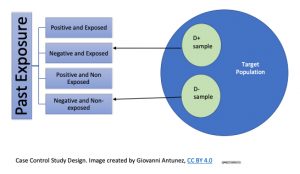 The study process requires, the identification of the individuals affected by the disease in study, which are called, ‘the cases,’ and their counterparts who are similar in characteristics (mainly demographics), which in this case are called, ‘control.’ So, a comparison between cases and controls is used to assess the risk associated with the development of a disease or, health problem. Since the data on the exposed (the cases), and the non-exposed (the controls) had happened in the past, the case-control study design is useful to assess prevalence but not incidence. Another characteristic for this type of study design is, that similar to the cross-sectional studies, the data collection is a single point in time, with the exception that in the case of case-control studies, the main characteristic is that the selection of the study subjects (participating) is based on the presence or, absence of the outcome.[14]
The study process requires, the identification of the individuals affected by the disease in study, which are called, ‘the cases,’ and their counterparts who are similar in characteristics (mainly demographics), which in this case are called, ‘control.’ So, a comparison between cases and controls is used to assess the risk associated with the development of a disease or, health problem. Since the data on the exposed (the cases), and the non-exposed (the controls) had happened in the past, the case-control study design is useful to assess prevalence but not incidence. Another characteristic for this type of study design is, that similar to the cross-sectional studies, the data collection is a single point in time, with the exception that in the case of case-control studies, the main characteristic is that the selection of the study subjects (participating) is based on the presence or, absence of the outcome.[14]
An example of a case control study is the work on a group of investigators who compared the Impact of windows and daylight exposure on overall health and sleep quality of office workers. The results showed that workers in windowless environments tend to experience limitations in their role in terms of physical problems and vitality and some sleep disturbances. When the two groups were compared, workers with windows had more light exposure, more physical activity, and longer sleep duration.[15] A selected image included in the article shows graphically how two (more variables were studied) of the characteristics between both groups show clearly the difference that makes to have windows or, not in the workplace.
 |
| “Windowless offices,” image from Wikimedia Commons, Licensed CC BY-SA 4.0 |
Common uses of the case-control study design
Because of all of the mentioned characteristics, the case control-study design is used to find the prevalence in the community of certain diseases, and from their results, a subsequent study is designed. Also, the case-control study design has been used especially for the study of rare diseases.
The selection of the cases and the controls in the case-control study design
This is an important step in the use of the case-control study design. The cases need to be selected based on a set of criteria, which defines the characteristics and manifestation of the disease, including laboratory and other medical tests such as imaging, including x-rays. Then, these criteria is used to identified the cases. And, what about the controls, how are those individuals found? The controls can be for example patients from the same clinic/hospitals as the cases, or, the same population, for example, college campus, or, factory workers. The major characteristic of the controls is the similarity in terms of the selection criteria used for the cases, so, a comparison can be established. When there is time to assess the risk, both groups cases and controls are included in the statistical calculations based on the exposed, and not exposed criteria and the development of the disease under study. It is important to note that the word, ‘exposed’ here is a matter of semantics, because the exposed do not necessarily existed, they are cases (they have the disease already).[16]
Cohort Study
This type of study design is considered the prototype (the model) of an almost ‘perfect’ design to investigate causality. In the cohort study, the main measure of disease frequency is, incidence. The following diagram presents how the study population is selected, and the major steps in the implementation of this type of study design:
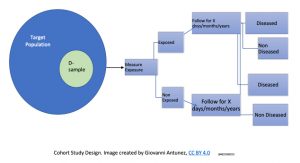
In the field of epidemiology is also accepted that cohort study address the issues of temporality (most studies do not) making possible to avoid logical errors. Cohort studies are usually conducted for longer periods of time compared to other designs; and the reason for it is that cohorts start with individuals who are free of the disease under study, and are followed up over the years to observe the development of this disease (or, group of diseases such as cardiovascular diseases). Due to the fact that most of the time, the data is collected in the future (from one specific point in time – the now, and the upcoming time of observation), the word retrospective is commonly used to reflect this concept, which makes most cohorts, examples of perspective studies.[17]
In some cases, a cohort study can be designed by using data that has already been collected (which resembles the case-control design), and since this is data collected in the past, then, the cohort is called, a retrospective cohort. And, when, retrospective data, present time data, and prospective data collection (which is essentially the classic cohort study) are included, the name of the cohort is, ambispective. But in reality most people when they hear the word, cohort, they are referring to prospective data collection studies (or, prospective cohorts).[18]
Cohort studies can be used to study more than one disease, or, multiple exposures, so, investigators can take some data (already collected in the cohort), and design a case-control study known as the ‘nested case-control,’ it is nested because it comes from inside the cohort.[19], [20]
Because of all of the mentioned benefits and advantages of cohorts, especially the calculation of incidence about a disease, makes cohorts an ideal study design, but at the same time it limitation is that cohorts are highly expensive, and that is mainly due to the fact that they last for a long time, especially for those diseases that take a long time to develop, so, the investigators have to wait for a while before they see the first generation of cases.[21]
Examples of Cohort Studies
To provide a mental picture of the cohort study design, I am including here, what I called, ‘Famous Cohorts in the United States.’ Famous because they reflect the reality of the country in terms of race segregation, and other socio-demographic factors that have shaped the country.
The Framingham Heart Study
The Framingham Heart Study takes its name from the town in which the study was conducted, Framingham. Framingham is located in Massachusetts, United States, and it within Middlesex County and the MetroWest subregion of the Greater Boston metropolitan area. [22] A picture of this city is shown below:
 |
| ‘Odd Fellows Building , Framingham MA’, image from Wikimedia Commons, licensed CC 3.0 Unported. |
The Framingham Heart Study is a classic example of a cohort study that assessed multiple exposures and multiple outcomes. This study, a collaboration between the US National Heart, Lung, and Blood Institute (a division of the National Institutes of Health) and Boston University, began in 1948 by enrolling just over 5,000 adults living in Framingham, Massachusetts. Investigators measured numerous exposures and outcomes, then repeated the measurements every few years. As the cohort aged, their spouses, children, children’s spouses, and grandchildren have been enrolled.[23]
The Framingham study is responsible for much of our knowledge about heart disease, stroke, and related disorders, as well as of the intergenerational effects of some lifestyle habits. [24] More information and a list of additional publications (more than 3,500 studies have been published using Framingham data) can be found extensively and especially in the project’s website. [25]
 |
| ‘Framingham Heart Study Physicians, 1948’, Image from Flickr. |
The Bogalusa Heart Study
Although, the Framingham Heart Study is a model cohort that influenced the work in public health and medicine. There was one flaw with the study, the participants were all white or, Caucasian; which from the beginning introduced a confounding factor that is race, which is a health determinant that is also linked to income, socio-economic status, social class, and among others, access to health services. So, to study cardiovascular disease beyond the white population generated the need to conduct a study on another major ethnic population group in the U.S. population, which is the black or African American community. Although not many studies on black, the Bogalusa Heart Study in Louisiana was born in 1972. Bogalusa is a small town in Louisiana (almost in the limit with Mississippi), it is mainly a biracial (black/white) rural community in which entire families have lived there for generations. The population in Bogalusa is mostly constant with few, or, no migration is ideal study population brought the attention of a famous Tulane University School of Public Health in New Orleans, Dr. Berenson, who lived through the entire duration of the study. [26],[27]. See a picture of the town, and also of Dr. Berenson:
 |
 |
|
| “Bogalusa City Hall“. Wikimedia Commons. Licensed CC BY-SA 4.0 | ‘Dr. Gerald Berenson’ the founder of the Bogalusa Heart Study, image from American Heart Association Journal. |
He survived the study which was taken down after hurricane Katrina due to major damage by the lack of electricity in New Orleans during Katrina in which many of the study samples that were stored were damaged, and also, the lack of funding after the impacts of Hurricane Katrina devastation in New Orleans in 2005.
The Bogalusa Heart Study started as an epidemiological study of cardiovascular risk factors in children and adolescents; it eventually evolved into observations of young adults. This study main milestones confirmed the findings of the Framingham Heart Study, but also superseed in terms of adding new variables to the study of cardiovascular disease, especially with the findings of the presence of cardiovascular disease in children, which had not been studied before. The study reported an African American child who died of cardiovascular disease and had atherosclerotic deposits in his arteries at the age of eight years old. This finding moved the American Heart Association to recommend that children stop been fed with whole cow’s milk after the child is 1; recommending 2% cow’s milk for children over 1 years old in the U.S. population. Another major milestone of the Bogalusa heart study is that identified several risk factors such as obesity, essential hypertension linked to kidney disease, and also how early onset of diabetes can also increase highly the development of cardiovascular disease at earlier ages, a finding that was also new to the medical and public health community.[28][29]
The San Antonio Heart Study
The San Antonio Heart Study conducted in Texas takes its name from this city. San Antonio, Texas, is a city of the Southern United States, and it is considered one of the seven most populous city in the U.S. [30]
 |
| ‘San Antonio, Texas’, image from Wikimedia, licensed CC BY-SA 4.0 |
The San Antonio Heart Study (SAHS), is another study that is no commonly mentioned in most epidemiology textbooks, and that brings another important perspective in the study of cardiovascular disease in the U.S. is the San Antonio Heart Study, which focused its efforts in identifying cardiovascular risk factors in the Latino population in the U.S. Again, as in the case of the Bogalusa Heart Study; the need to study in detail what happened to another major ethnic group in the U.S. was critical, what was found among Caucasians or, Whites in the U.S. cannot necessarily be applied (extrapolated) to the African American community, nor, to the Latino community, so, the study was justified. And its findings among others discovered that the Latino heart is hard to die. Among all of the major ethnic groups in the U.S., Latinos have the lowest rates of heart attacks after accounting for several confounding factors.[31],[32],[33]
Note: the above content about cohorts had presented the problem of heart disease among the white, black and Latino population in the U.S. but there is literature available about other ethnic groups in the U.S. such as Native Americans, Asians, and Pacific Islanders, however, those studies are not cohort studies, only reports about the health status of these mentioned groups, for example, there is one report about Native Americans in the U.S. [34]
Cohort Studies major disadvantages and historical ‘mistakes’
Besides the disadvantage already mentioned before in the content, that cohorts are very expensive (they usually costs millions over the years of duration), it is not possible to have a cohort for every disease that exist, or, that is highly prevalence in the population. There is another limitation of cohorts, they cannot be used for the study of rare diseases, because one of the characteristics of a rare disease is that it is not suffered by a great number of people in the population, making the sample usually small, or, it may take long times to get enough data that can be used meaningfully in the practice.
Another major disadvantage of cohorts, which is the reason I included the examples of the ‘famous cohorts in the U.S.,’ is that in this country, cohorts were linked to the problem of racial preference, the first ethnic group represented in a popular cohort such as the Framingham heart study is the white population, which excluded the other ethnic groups in the U.S. who also are affected by heart disease. This fact is an example of the historical exclusion of people of color and indigenous populations in the U.S., so, the history of major cohorts in the U.S. is also reflecting the need to study those oppressed, and ignored throughout history. Another observation in this context is, that for example, the Bogalusa Heart Study, and the San Antonio Heart Study are not well known in the scientific community, teaching medical schools, and other similar educational institutions do not know – or, pretend to not knowing about the existing of these studies, which provides extremely important data for the prevention of coronary heart disease in the nation.
Clinical Trials
Clinical Trials
They are considered the highest level of the research designs discussed above in this chapter, and they are very much the standard design used especially for pharmaceutical companies to assess the effectiveness and safety of drugs, certain medical procedures, sophisticated medical equipment, etc. There at least two types of clinical trials as it was mentioned in the introduction of this chapter in the summary of types of study design; 1) Preventive or, Community Trials, and 2) Therapeutic clinical trials. The discussion in this chapter will be mainly focused in the second group or, therapeutic clinical trials, also, just called, clinical trials.
Community (or, preventive) trials
These type of studies are used to determine the potential benefit of new policies and programs. They are called, community because it refers to the population, or, specific groups in the population. In general, the community trials will evaluate the impact of specific interventions that intent to produce changes in a target population. For example, the knowledge, attitudes and practices related to the Medicare program; or, the use (by the target population) of health care services to prevent and treat heart disease, etc.
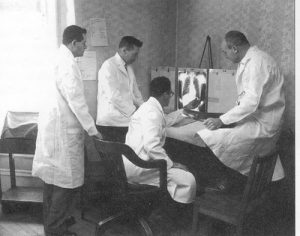
The first step in the process of a community trial is to determine eligible communities, or, groups, and their willingness to participate. Then, baseline data is collected, this type of information can be for example, target population demographics, cultural traits, data from the national census, disease rates, etc., of the problem to be addressed in the intervention, and the collected information is also used for the control communities. In addition, the trial participants are selected by randomization (which is described in more details later in this section) and the selected individuals (or, groups) are followed over time. Data is entered, analyzed, and reports generated. Finally, the outcomes of interest are measured and used to assess the effectiveness, or, to identified weak points in the program intervention, and how to improve the quality of the programs and services offered to the target population, or, group. An example of a community trial and its protocol is summarized in the image below:
 |
| ‘Design of rural community-based trial part of the study’. CLBD: chronic low back disorder; NP: nurse practitioner; PT: physical therapist. Image from Research Gate. |
Advantages and disadvantages of community trials
The major advantages of community trials is that are unique in providing information that be can used to estimate the impact of change in the behavior or modifiable exposure of the incidence of disease in a community or, group; and also, the effectiveness of services and programs offered to the target group. As any other study design, the community trials have some also some disadvantages, for example, in general they are considered inferior to clinical (therapeutic) trials – discussed in detail in the rest of this chapter); and that is because selection of participants into the study, delivery of the intervention, and monitoring of the study outcomes are not as strict (or, rigorous) as it is in a clinical trial. Other disadvantages is that the study results are affected by some population dynamics, especially secular trends because of the mobility, or, changes in the target population. Also, it is hard to avoid the influence of non intervention forces surrounding the study population or, group.
Clinical trials
Since the content above has been mostly about the clinical community (or, preventive) trials. The information that follows will focus mainly in the conduction of therapeutic clinical trials, commonly called just, ‘clinical trials.’ What are clinical trials? A common definition is that, clinical trials are planned experiments that assesses the efficacy of a treatment (or, medical procedure) in people. It is medical research involving people. In a clinical trial, the study outcomes in a treated group are compared with outcomes in an equivalent control group. Participants in both groups are enrolled, treated, and followed over the same time period. [35]
Methods commonly used in clinical trials
There is a series of methods or, strategies used for clinical trials, and these are at the same time considered the major strengths of clinical trials. The most important are discussed in the following paragraphs.
Study Protocol
The clinical trials protocol is usually an extensive and detailed manual that outline the major steps of the study, especially outlining what could happen in certain situations during the completion of the study. For example, what to do is the investigators deviate from the originally planned study assignments of the participants? How many deviations in the protocol would be allowed? It is customary that for example, no more than three deviations would be allowed during the duration of the trial. Also, the protocol include the data collection instruments, data input procedures, and analyses once the data is collected. The presence of a protocol is a valuable tool to assure that the clinical trial is conducted under the required academic and scientific rigor.
One of the major elements of the mentioned document is to plan ahead for any deviation of the study protocol. To prevent for this to happen, planned crossovers are part of the protocol. In this case, the study participant may server as his/her own control. And, when unplanned situations occurs for example a change of treatment is requested by a study participant; this change is called, an unplanned crossover, which could exist in for example situations in which the study participant request a change of treatment. It is recommended that no many unplanned crossovers occur during the duration of the trial, because it can compromise the study results, and the quality of the study in general.
Selection of the study participants
One of the methods (and strengths) of clinical trials is the careful selection of study participants. For this purpose, research in clinical trials used what is known as randomization, a statistical method to sort the possible study participants before they can participate in the study. Essentially, the randomization procedure allows to use random selection twice, for example, a person is assigned a number that will be randomly picked for the study participation, and then, if selected, the person (now, a study participant) will be randomly assigned to the drug, procedure, placebo or, no treatment branch of the trial.
Randomization is the preferred method for assigning subjects to the treatment or control conditions of a clinical trial. If not random assignment is used then, mixing of effects of the intervention can occur, which at the same time, create differences among the study participants in the trial.
Blinding
An additional method or strategy commonly used in clinical trials is also one of the major strengths of clinical trials is that they control bias, especially selection bias. And, to control for this, the procedure known as blinding is used. At least three types of blinding are known, 1) single blinding, it is when the study participants (clients, patients) don’t know about the type of drug, or, medical procedure they have been assigned, or, they don’t know if the treatment they are receiving is placebo. 2) double blinding, it is when the client/patient doesn’t know what type of treatment or, placebo they are receiving (the clients/patient), or, the health care providers (doctors, nurses, technicians, etc.) are administering. 3) triple blind, the client/patient, the health care providers, nor the data collection and analysis have knowledge of the treatment given to the study participants. Additional blinding strategies can be used, but the mentioned here are the most commonly used.
Phases of clinical trials
 |
| ‘Clinical trials phases’, image from JLI (James Lind Institute). |
These phases can be observed for example during the creation and testing of a new vaccine process follows:
Phase II: expands testing to a group of 100 to 200 subjects (from the targeted population, or group).
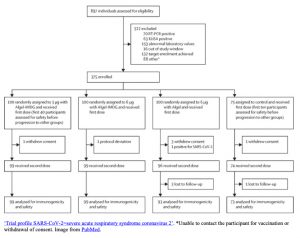
There is a document called, the Consort Statement (a flowchart) that is a protocol used to guide the reporting of randomized trials by providing a 22-item checklist and a flowchart. The form is presented below:
 |
|
Strengths and limitations of clinical trials
The major strengths of clinical trials is for the investigator to have the greatest control over the amount of exposure, the timing and the frequency of that exposure, and the observation period. Also, the use of randomization greatly reduces the likelihood that groups will differ significantly, which is of enormous benefit to the enhancement of the results. The limitations of clinical trials include ethical dilemmas such as how the benefits would outweigh the risk, how to protect the interests of the study participants and not only the investigators, when to stop a trial if a major adverse health outcome occurs during the completion of the study, and overall, how much of the information in the trial is shared with the study participant in the informed consent form.
Summary
This chapter has covered the most common epidemiology study designs and its uses. These designs include: the case study/case reports, ecologic study, cross-sectional, case controls, cohort studies, and clinical trials. When they are classified by type of study, they can be descriptive or, analytic studies. The most common descriptive studies are, the case report/case series, ecologic and cross sectional studies. Analytic studies, they can be classified into intervention and observational. Examples of analytic ‘intervention’ studies are, community (preventive) trials and clinical (therapeutic) trials. And, observational studies, examples, the case control, and the cohort studies. The use of these mentioned study designs depending of the research questions, the purposes of the study, and the availability of resources to conduct them. Investigators always look for those study designs that are relatively quick, less expensive, and efficient.
- No Author. (n.d.). Introduction to study designs - geographical studies, available at: https://www.healthknowledge.org.uk/e-learning/epidemiology/practitioners/introduction-study-design-gs ↵
- Levac, D., Colquhoun, H., & O'Brien, K. K. (2010). Scoping studies: advancing the methodology. Implementation science : IS, 5, 69. https://doi.org/10.1186/1748-5908-5-69 ↵
- P Sai Kumar, Imperial College London.(n.d.). Epidemiology for Practitioners. Resource text adapted from material written by Maria Kirwan, available at www.healthknowledge.org.uk ↵
- community medicine, study designs, howmed net.: HYPERLINK "http://howmed.net/community-medicine/study-designs/" o "Figure: Study designs" t "_blank" http://howmed.net/community-medicine/study-designs/ ↵
- Georgia State University Library. (n.d.). Research Guides, Literature Reviews and Types of Clinical Study Designs. From https://research.library.gsu.edu/c.php?g=115595&p=755213 Last Updated: Mar 11, 2022 2:42 PM ↵
- Deakin University Library. (n.d.). Quantitative Study Designs: Case Study / Case Report / Case Series. From https://deakin.libguides.com/quantitative-study-designs/casestudy ↵
- Kooistra, B., Dijkman, B., Einhorn, TA., Bhandari, M. (May 01, 2009). How to Design a Good Case Series, The Journal of Bone & Joint Surgery: 91(3), 21-26. From https://journals.lww.com/jbjsjournal/fulltext/2009/05003/How_to_Design_a_Good_Case_Series.5.aspx doi: 10.2106/JBJS.H.01573 ↵
- Freedman, DA. (199). Ecological Inference and the Ecological Fallacy. Department of Statistics, University of California Berkeley, available at https://web.stanford.edu/class/ed260/freedman549.pdf ↵
- LaMorte, W.W. (2020). Death rates of coronary heart disease (CHD) in different countries In PH717 Module 1B, Descriptive Tools, Descriptive Epidemiology & Descriptive Statistics. Boston University School of Public Health. From https://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH717-QuantCore/PH717-Module1B-DescriptiveStudies_and_Statistics/PH717-Module1B-DescriptiveStudies_and_Statistics6.html ↵
- Freedman, DA. (199). Ecological Inference and the Ecological Fallacy. Department of Statistics, University of California Berkeley, available at https://web.stanford.edu/class/ed260/freedman549.pdf ↵
- British Medical Journal (BMJ). (n.d.). Chapter 6. Ecological studies (Geographical Comparisons, Time Trends, Migrants, Occupation and Social Class) in Epidemiology for the uninitiated. From https://www.bmj.com/about-bmj/resources-readers/publications/epidemiology-uninitiated/6-ecological-studies#chapters ↵
- Setia M. S. (2016). Methodology Series Module 3: Cross-sectional Studies. Indian journal of dermatology, 61(3), 261–264. https://doi.org/10.4103/0019-5154.182410 ↵
- Olds, T.S., Maher, C.A., Ridley, K. et al. (2010). Descriptive epidemiology of screen and non-screen sedentary time in adolescents: a cross sectional study. Int J Behav Nutr Phys Act 7(92). From https://doi.org/10.1186/1479-5868-7-92 ↵
- Tenny, S., Kerndt, C. C., & Hoffman, M. R. (2022). Case Control Studies. In StatPearls. StatPearls Publishing. From https://pubmed.ncbi.nlm.nih.gov/28846237/ ↵
- C. H., & Zee, P. C. (2014). Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. Journal of clinical sleep medicine, 10(6), 603–611. From https://doi.org/10.5664/jcsm.3780 ↵
- Setia M. S. (2016). Methodology Series Module 2: Case-control Studies. Indian journal of dermatology, 61(2), 146–151. https://doi.org/10.4103/0019-5154.177773 ↵
- Song, J. W., & Chung, K. C. (2010). Observational studies: cohort and case-control studies. Plastic and reconstructive surgery, 126(6), 2234–2242. https://doi.org/10.1097/PRS.0b013e3181f44abc ↵
- School of Public Health, Boston University. (n.d.). Cohort Studies. From https://sphweb.bumc.bu.edu/otlt/mph-modules/ep/ep713_cohortstudies/ep713_cohortstudies_print.html ↵
- Ernster V. L. (1994). Nested case-control studies. Preventive medicine, 23(5), 587–590. https://doi.org/10.1006/pmed.1994.1093 ↵
- Soyoung K. (2016). Case-Cohort Studies vs Nested Case-Control Studies. Datum, Newsletter of Biostatistics, Medical College of Wisconsin (MCW). 22(1). From https://www.mcw.edu/-/media/MCW/Departments/Biostatistics/vol22no1kim.pdf?la=en ↵
- School of Public Health, Boston University. (n.d.). Advantages & Disadvantages of Cohort Studies. From https://sphweb.bumc.bu.edu/otlt/mph-modules/ep/ep713_cohortstudies/EP713_CohortStudies5.html ↵
- Framingham, Massachusetts, Wikipedia, From https://en.wikipedia.org/wiki/Framingham,_Massachusetts ↵
- No Author. (n.d.). Framingham Heart Study, Participant cohorts. Available at https://framinghamheartstudy.org/participants/participant-cohorts/ ↵
- Framingham Heart Study. (2019). Participant Cohorts. From https://framinghamheartstudy.org/participants/participant-cohorts/ ↵
- Framingham Heart Study. (n.d.) The Framingham Heart Study, Available at http://www.framinghamheartstudy.org/ ↵
- Wikipedia. (n.d.). Gerald Berenson. From https://en.wikipedia.org/wiki/Gerald_Berenson ↵
- No Author. (n.d.). Bogalusa Heart Study. Available at: https://www.clersite.org/bogalusaheartstudy/ ↵
- No author. (n.d.). Bogalusa Heart Study. From https://www.clersite.org/bagalusaheartstudy/ ↵
- Berenson G. S. (2001). Bogalusa Heart Study: a long-term community study of a rural biracial (black/white) population. The American journal of the medical sciences, 322(5), 267–274. ↵
- San Antonio, Texas. Wikipedia. From https://en.wikipedia.org/wiki/San_Antonio ↵
- Shen D, Mitchell B, Hazuda H, Clark G, and Stern M. (1992). The San Antonio heart study research information study, Proceedings Computers in Cardiology, 607-610. Available at: https://ieeexplore.ieee.org/document/269385/keywords#keywords ↵
- Haffner, S. (2000). Obesity and the metabolic syndrome: The San Antonio Heart Study. British Journal of Nutrition, 83(S1), S67-S70. From https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/obesity-and-the-metabolic-syndrome-the-san-antonio-heart-study/1E337C3FA2476672AD426FB90209EC10 ↵
- Haffner SM, Miettinen H, Gaskill SP, Stern MP. (1996). Metabolic Precursors of Hypertension: The San Antonio Heart Study. Arch Intern Med. 156(17):1994–2001. doi:10.1001/archinte.1996.00440160106013 Access to abstract from https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/622459 ↵
- Breathett, K., Sims, M., Gross, M., Jackson, EA., Jones, EJ et al. (2020). Cardiovascular Health in American Indians and Alaska Natives: A Scientific Statement from the American Heart Association. Circulation, 141:e948–e959. From https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000773 ↵
- National Institute of Aging. (n.d.). What Are Clinical Trials and Studies? From https://www.nia.nih.gov/health/what-are-clinical-trials-and-studies ↵
observations made at the group level may not represent the exposure-disease relationship at the individual level
in epidemiology this term is used to refer to 'time' for example, the changes in disease prevalence over time.
the term refers to a defined unit, for example, a county, state, or, school district, etc.
Any program or other planned effort designed to produce changes in a target population. For example, health care use, etc.
essentially randomly selecting a study participant twice.
clinical trials are planned experiments that assesses the efficacy of a treatment (or, medical procedure) in people. It is medical research involving people.
Any planned, or, unplanned deviation of the study protocol.
To 'blind' the study participants, or, the providers or, both by not knowing to what clinical intervention of drug they are assigned or, participating, and it is commonly used in clinical trials.

Feedback/Errata