10.4 The Atmosphere
The Atmosphere
Earth’s atmosphere is a thin blanket of gases and tiny particles—together called air. We are most aware of air when it moves and creates wind. All living things need some of the gases in air for life support. Without an atmosphere, Earth would likely be just another lifeless rock. Earth’s atmosphere, along with the abundant liquid water at Earth’s surface, are the keys to our planet’s unique place in the Solar System. Much of what makes Earth exceptional depends on the atmosphere. Let’s consider some of the reasons we are lucky to have an atmosphere.
Without the atmosphere, Earth would look a lot more like the Moon. Atmospheric gases, especially carbon dioxide (CO2) and oxygen (O2), are extremely important for living organisms. In photosynthesis, plants use CO2 and create O2. Photosynthesis is responsible for nearly all of the oxygen currently found in the atmosphere. By creating oxygen and food, plants have made an environment that is favorable for animals. In respiration, animals use oxygen to convert sugar into food energy they can use. Plants also go through respiration and consume some of the sugars they produce.
The relationship between the atmosphere and water vapor is a crucial part of the hydrologic cycle. Water spends a lot of time in the atmosphere, mostly as water vapor. All weather takes place in the atmosphere, and virtually all of it happens in the lower atmosphere. The amount of water vapor present in different areas of the planet has a dynamic effect on the weather that occurs in different areas. Areas that are prone to regularly occurring water-rich storms have quite different ecosystems than drier areas that receive less moisture. Species are adapted to the conditions in which they live. Drier areas may have species of plants and animals that have strategies that allow them to thrive, whereas more temperate areas with more moisture have species that would not fare as well in drier climates. Thus, life depends on the climate and weather that occur in the zones in which life occurs.
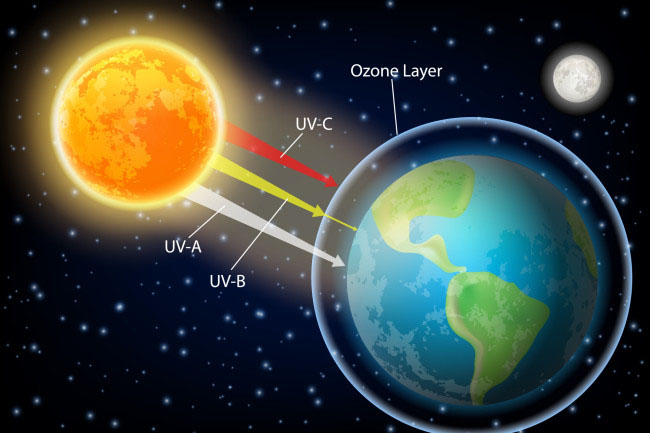
Ozone is a molecule composed of three oxygen atoms (O3). Ozone in the upper atmosphere absorbs high-energy ultraviolet (UV) radiation coming from the Sun. This protects living things on Earth’s surface from the Sun’s most harmful rays. Without ozone for protection, only the simplest life forms would be able to live on Earth.
Along with the oceans, the atmosphere keeps Earth’s temperatures within an acceptable range. Greenhouse gases trap heat in the atmosphere, so they help to moderate global temperatures. This is referred to as the greenhouse effect. You can observe this on a sunny day when you walk into a room with a lot of windows and no air conditioning. The room is very warm. Without an atmosphere with greenhouse gases, Earth’s temperatures would be frigid at night and scorching during the day. Important greenhouse gases include carbon dioxide, methane, water vapor, and ozone.
Atmospheric Gases
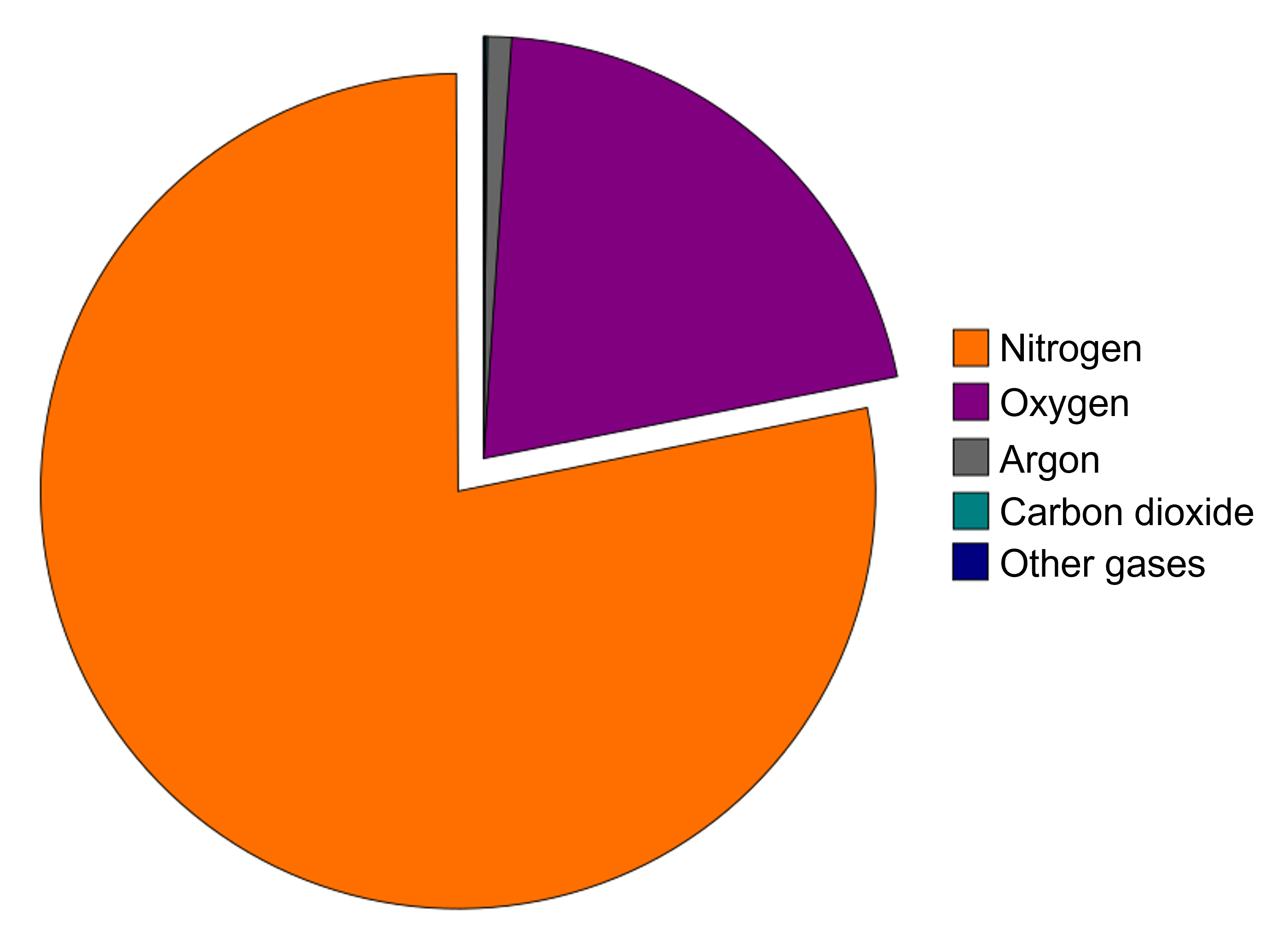
Nitrogen and oxygen together make up 99 percent of the planet’s atmosphere. The rest of the gases are minor components but sometimes are very important. Humidity is the amount of water vapor in the air. Humidity varies from place to place and season to season. This fact is obvious if you compare a summer day in Atlanta, Georgia, where humidity is high, with a winter day in Phoenix, Arizona, where humidity is low. When the air is very humid, it feels heavy or sticky. Dry air usually feels more comfortable. Higher humidity is found around the equatorial regions because air temperatures are higher and warm air can hold more moisture than cooler air. Of course, humidity is lower near the polar regions because air temperature is lower.
Some of what is in the atmosphere is not gas. Particles of dust, soil, fecal matter, metals, salt, smoke, ash, and other solids make up a small percentage of the atmosphere. Particles provide starting points (or nuclei) for water vapor to condense on and form raindrops, and some particles are pollutants.
The atmosphere has different properties at different elevations above sea level, or altitudes. The air density (the number of molecules in a given volume) decreases with increasing altitude. This is why people who climb tall mountains, such as Mount Everest, have to set up camp at different elevations to let their bodies get used to the decreased air density.
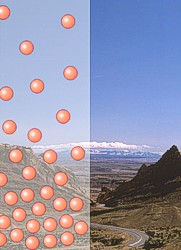
Why does air density decrease with altitude? Gravity pulls the gas molecules toward Earth’s center. The pull of gravity is stronger at sea level due to being closer to the center of the Earth than at higher altitudes. Air is denser at sea level where the gravitational pull is greater. Gases at sea level are also compressed by the weight of the atmosphere above them. The force of the air weighing down over a unit of area is known as its atmospheric pressure. The reason why we are not crushed by this weight is because the molecules inside our bodies are pushing outward to compensate. Atmospheric pressure is felt from all directions, not just from above.
At higher altitudes, the atmospheric pressure is lower and the air is less dense than at higher altitudes. If your ears have ever “popped,” you have experienced a change in air pressure. Gas molecules are found inside and outside your ears. When you change altitude quickly, like when an airplane is descending, your inner ear keeps the density of molecules at the original altitude. Eventually the air molecules inside your ear suddenly move through a small tube in your ear to equalize the pressure. This sudden rush of air is felt as a popping sensation.
Although the density of the atmosphere changes with altitude, the composition stays the same with altitude, with one exception. In the ozone layer, at about 20 km to 40 km above the surface, there is a greater concentration of ozone molecules than in other portions of the atmosphere.
Layers of the Atmosphere
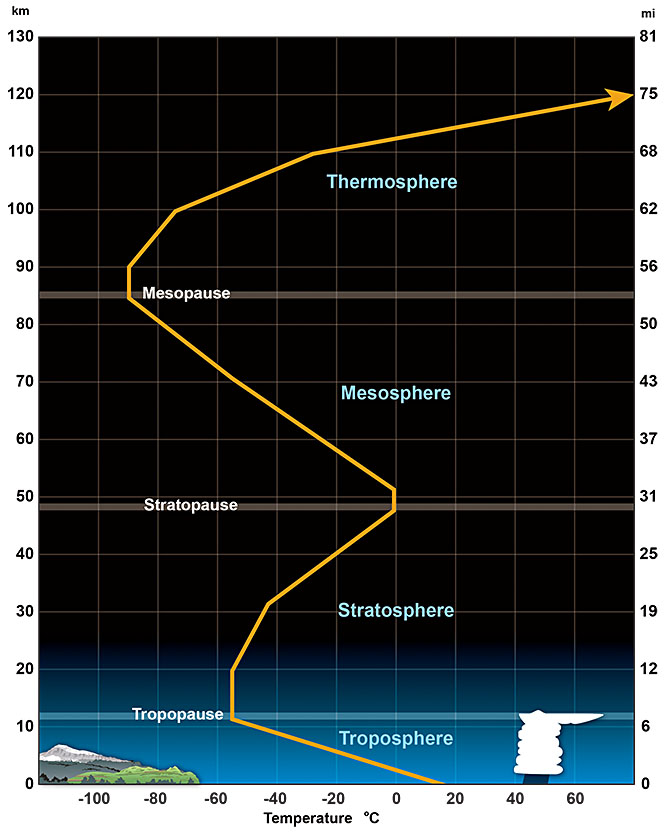
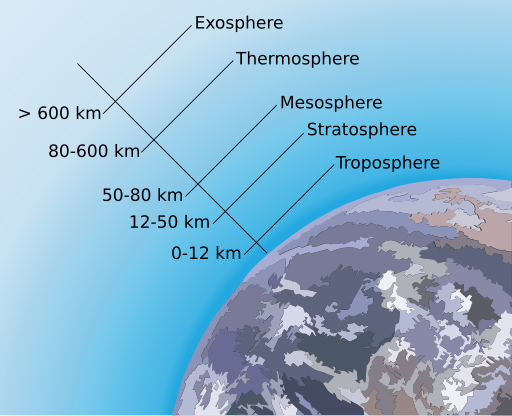
The atmosphere’s layers correspond with how the atmosphere’s temperature changes with altitude. By understanding the way temperature changes with altitude, we can learn a lot about how the atmosphere works. While weather takes place in the lower atmosphere, interesting things, such as the aurora, happen higher in the atmosphere.
Warm air rises because gas molecules are able to move freely in the atmosphere. When gas molecules are warm, they move vigorously and take up more space. When gas molecules are cool, they are sluggish and do not take up as much space. With the same number of molecules in less space, both air density and air pressure are higher. Warmer, lighter air is more buoyant than the cooler air above it, so it rises. The cooler air then sinks down because it is denser than the air beneath it. This is the process of convection.
The property that changes most strikingly with altitude is air temperature. Unlike the change in pressure and density, which decrease with altitude, changes in air temperature are not regular. A change in temperature with distance is called a temperature gradient. The atmosphere is divided into layers based on how the temperature in that layer changes with altitude, which is known as the layer’s temperature gradient. In some layers, temperature increases with altitude, and in others it decreases. The temperature gradient in each layer is determined by the heat source of the layer. Most of the important processes of the atmosphere take place in the lowest two layers: the troposphere and the stratosphere.
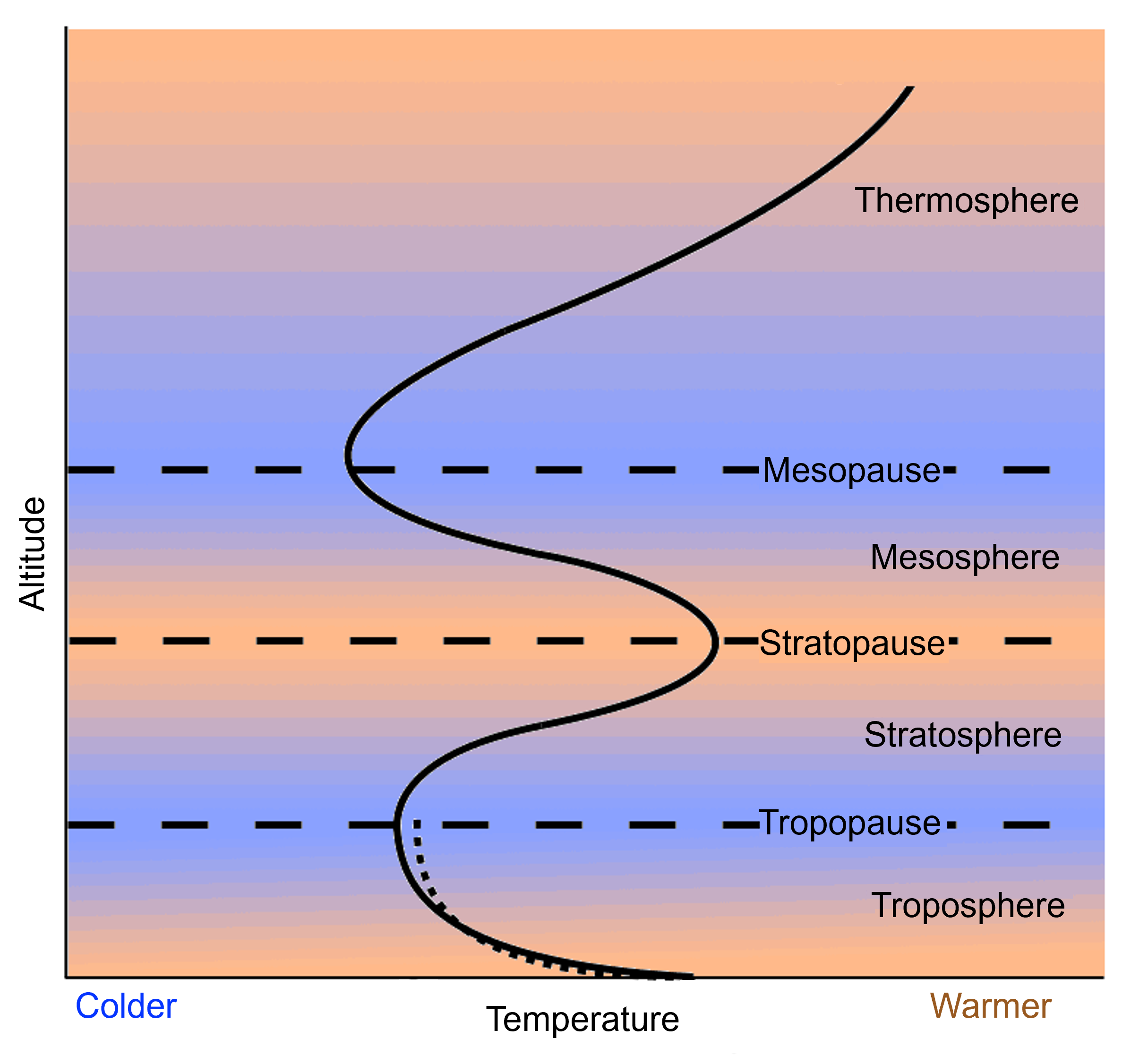
The temperature of the troposphere is highest near the surface of the Earth and decreases with altitude. On average, the temperature gradient of the troposphere is 6.5°C per 1,000 m (3.6°F per 1,000 ft) of altitude. Earth’s surface is a major source of heat for the troposphere, although nearly all of that heat comes from the Sun. Rock, soil, and water on Earth absorb the Sun’s light and radiate it back into the atmosphere as heat. The temperature is also higher near the surface because of the greater density of gases. In the troposphere, warmer air is beneath cooler air, a condition that is unstable. The warm air near the surface rises while cool air higher in the troposphere sinks, resulting in mixing. This mixing causes the temperature gradient to vary with time and place. The rising and sinking of air in the troposphere also means that nearly all of the planet’s weather takes place in the troposphere.
Atmospheric Energy, Temperature, and Heat
Energy travels through space or material. This is obvious when you stand near a fire and feel its warmth or when you feel heat in the handle of a metal pot even though the handle is not sitting directly on the hot stove. Invisible energy waves can travel through air, glass, and even the vacuum of outer space. These waves have electrical and magnetic properties, so they are called electromagnetic waves. The transfer of energy from one object to another through electromagnetic waves is known as radiation. Different wavelengths of energy create different types of electromagnetic waves.
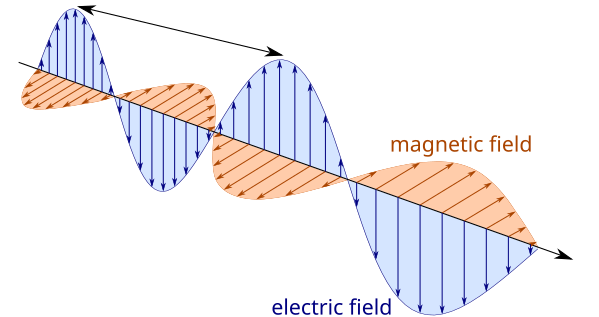
The wavelengths humans can see are known as visible light. These wavelengths appear to us as the colors of the rainbow. The longest wavelengths of visible light appear red. Infrared wavelengths are longer than visible red. We feel infrared energy as heat. Wavelengths that are shorter than violet are called ultraviolet.
Reflection is when light (or another wave) bounces back from a surface. Albedo is a measure of how well a surface reflects light. A surface with high albedo, for example a snow field, reflects a large percentage of light. One important fact to remember is that energy cannot be created or destroyed; it can only be changed from one form to another. This is such a fundamental fact of nature that it is a law: the law of conservation of energy. In photosynthesis, for example, plants convert solar energy into chemical energy that they can use. They do not create new energy. When energy is transformed, some nearly always becomes heat. Heat transfers from warmer objects to cooler ones easily. If no more heat is added, eventually all of a material will reach the same temperature.
Temperature is a measure of how fast the atoms in a material are vibrating. High temperature particles vibrate faster than low temperature particles. Rapidly vibrating atoms smash together, which generates heat. As a material cools down, the atoms vibrate more slowly and collide less frequently. As a result, they emit less heat. Temperature measures how fast a material’s atoms are vibrating, while heat measures the material’s total energy.
Heat is taken in or released when an object changes state, changing from a gas to a liquid or a liquid to a solid. This heat is called latent heat. When a substance changes state, latent heat is released or absorbed. A substance changing its state of matter does not change temperature. All of the energy that is released or absorbed goes toward changing the material’s state. Substances also differ in their specific heat, which is the amount of energy needed to raise the temperature of one gram of the material by 1°C (1.8°F). Water has a very high specific heat, which means it takes a lot of energy to change the temperature of water.
Energy from the Sun
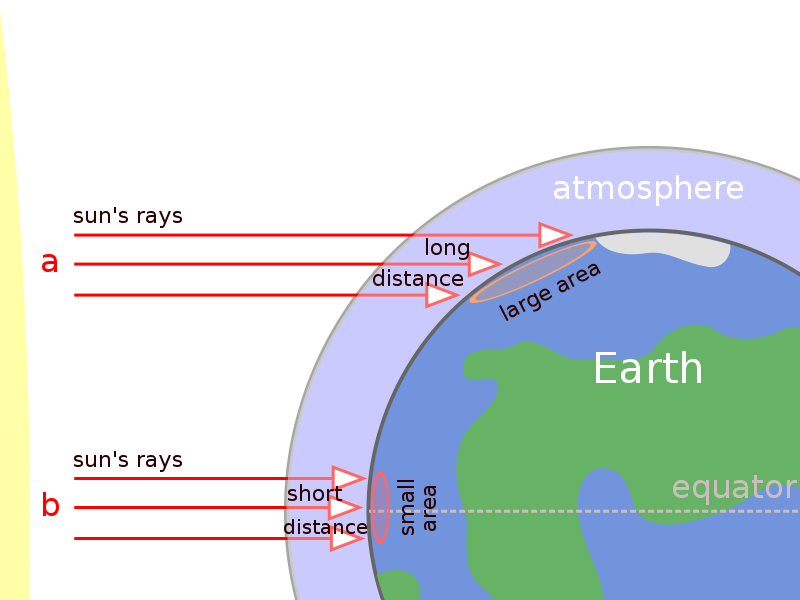
The Earth constantly tries to maintain an energy balance with the atmosphere. Most of the energy that reaches the Earth’s surface comes from the Sun. About 44% of solar radiation is in the visible light wavelengths, but the Sun also emits infrared, ultraviolet, and other wavelengths. When viewed together, all of the wavelengths of visible light appear white. Of the solar energy that reaches the outer atmosphere, UV wavelengths have the greatest energy. Only about 7% of solar radiation is in the UV wavelengths. The remaining solar radiation is the longest wavelength, infrared. Most objects radiate infrared energy, which we feel as heat.
Heat moves in the atmosphere the same way it moves through the solid Earth or another medium. Radiation is the transfer of energy between two objects by electromagnetic waves. Heat radiates from the ground into the lower atmosphere.
In conduction, heat moves from areas of more heat to areas of less heat by direct contact. Warmer molecules vibrate rapidly and collide with other nearby molecules, transferring their energy. In the atmosphere, conduction is more effective at lower altitudes, where air density is higher; this transfers heat upward to where the molecules are spread farther apart or transfers heat laterally from warmer to cooler spots, where the molecules move less vigorously. Heat transfer by movement of heated materials is called convection. Heat that radiates from the ground initiates convection cells in the atmosphere.
Greenhouse Gases and the Greenhouse Effect
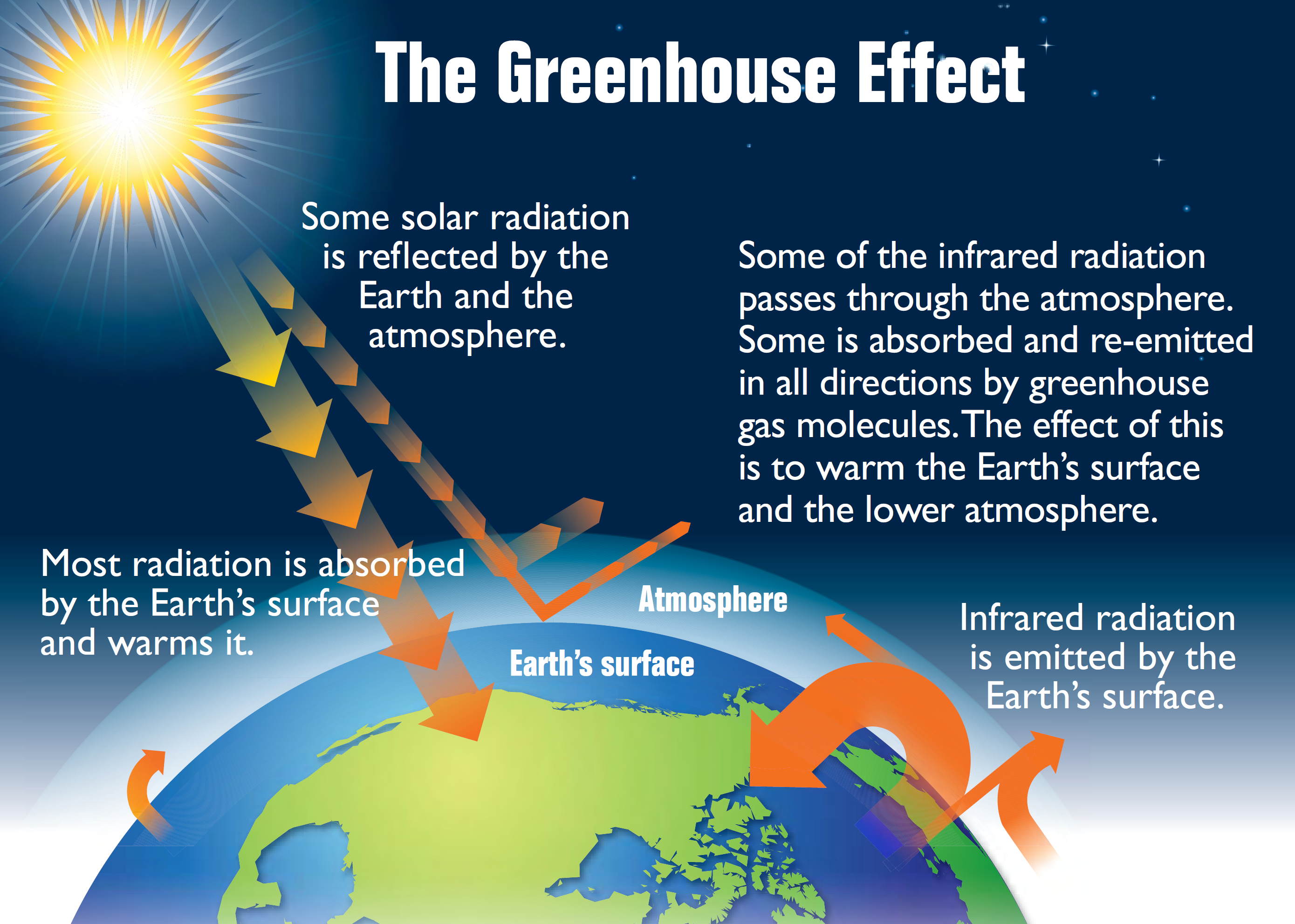
Earth is not getting warmer or cooler due to incoming solar radiation, which means that the planet’s heat budget is in balance, although the amount of incoming solar energy is different at different latitudes. The difference in solar energy received at different latitudes drives atmospheric circulation. The exception to Earth’s temperature being in balance is caused by greenhouse gases. Greenhouse gases warm the atmosphere by trapping heat. Some of the heat radiation from the ground is trapped by greenhouse gases in the troposphere. Like a blanket on a sleeping person, greenhouse gases act as insulation for the planet. The warming of the atmosphere because of insulation by greenhouse gases is called the greenhouse effect. Greenhouse gases are the component of the atmosphere that moderate Earth’s temperatures. Greenhouse gases include carbon dioxide (CO2), water vapor (H2O), methane (CH4), ozone (O3), nitrous oxides (N2O), and chlorofluorocarbons (CFCs). All of these gases are a normal part of the atmosphere except manmade CFCs.
Different greenhouse gases have different abilities to trap heat. For example, one methane molecule traps 30 times as much heat as one carbon dioxide molecule. One CFC-12 molecule (a type of CFC) traps 10,600 times as much heat as one CO2 molecule. Still, CO2 is a very important greenhouse gas because it is much more abundant in the atmosphere. Human activity has significantly raised the levels of many of greenhouse gases in the atmosphere. Methane levels are about 2.5 times higher as a result of human activity. Carbon dioxide has increased more than 35%. CFCs have only recently existed. This is a concern because, as atmospheric greenhouse gas levels increase, more greenhouse gases trap more heat and warm the atmosphere. The increase or decrease of greenhouse gases in the atmosphere affect climate and weather over the entire Earth.
Take this quiz to check your comprehension of this section.

Attribution:
This section is cloned from Introduction to Earth Science, Second Edition, by Laura Nese
r, Virginia Tech, published using Pressbooks, under a CC BY-NC.SA 4. (Attribution-NonCommercial-ShareAlike 4.0 International; https://creativecommons.org/licenses/by-nc-sa/4.0/). It may differ from the original.
Media Attributions
- Figure-3.-Species-diversity-is-very-high-in-tropical-wet-forests-1
- Figure-5.-Although-savannas-are-dominated-by-grasses-small-woodlands-such-as-this-one-in-Mount-Archer-National-Park-in-Queensland-Australia-may-dot-the-landscape-1
- freshwater-resources-pitcher
- Section 14.1 quiz
